Your cart is currently empty!
The Atlas of Science (atlasofscience.org) is a research oriented newsletter whose goal is to promote the dissemination of scientific research.
10/07/2020: The Atlas of Science featured an article covering AlphaThera’s proprietary method of Site-specific antibody labeling through the use of photo-crosslinking technology. The article presents a layman’s summary of how the novel, site-specific approach to labeling antibodies improves upon traditional methods of antibody conjugation. The method is widely compatible and modular, enabling labs to rapidly label their antibodies for improved assay performance. Read the full description below of the LASIC: Light Activated Site-Specific Conjugation of Native IgGs method.
Site-specific antibody labeling with novel Photo-crosslinking Technology
Layman’s Summary Featured in Atlasofscience.org
Our research presents a novel, site-specific approach to labeling antibodies with molecules for various applications, improving upon traditional methods of antibody conjugation. The method is widely compatible and modular, enabling labs to rapidly label their antibodies for improved assay performance.
Antibodies are highly-specific, Y-shaped proteins that serve as versatile tools for research, diagnostic and therapeutic applications. The two arms of the antibody are able to seek and bind cellular and molecular targets. Researchers have long harnessed the specific binding capability of antibodies for various purposes. For example, antibodies can be labeled with cargo, such as drugs or fluorescent dyes, acting as a carrier that directs the cargo to specific cell populations. While methods used to label or ‘conjugate’ antibodies have long been established, these traditional methods have limitations that impact the performance of antibodies in assays and drug delivery.
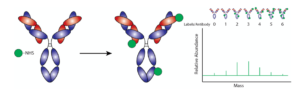
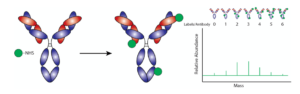
Fig. 1. Traditional Antibody Labeling Methods: Notice how the non site-specific labeling method, using NHS-ester chemical groups, attaches the cargo (green) randomly on the antibody. The chart on the right shows how the number of labels per antibody is highly variable, yielding a heterogeneous mix of product. This can significantly reduce antibody performance.
Labeling antibodies with cargo or other functional molecules can be accomplished through a variety of chemical methods that link these molecules to specific amino acids that are naturally present on the antibody surface. However, these common labeling methods are not “site-specific”, meaning that the cargo molecules are randomly attached to the antibody, including to sites near or even within the antibody’s binding region (Fig. 1). This can interfere with antibody binding to its target and results in a heterogeneous mix of antibody conjugates. The end result of non site-specific labeling is lower assay sensitivity and specificity.
Our research presents a novel approach to conjugate antibodies by using photo-reactive linker molecules that site-specifically and uniformly bind the Fc-constant region of the antibody, which vastly improves the antibody’s binding ability and performance. These small linker molecules are known as photo-reactive antibody binding domains (pAbBD) or the trade name oYo-LinkTM (AlphaThera, LLC). The pAbBDs are either expressed as a fusion protein with the desired cargo of interest or can be generated with a unique reactive chemical handle at the c-terminus that allows for the precise attachment of the desired cargo. These linker molecules, when exposed to 365 nm ‘black’ light, will covalently attach onto the Fc-region of nearly any existing, “off-the-shelf” antibody. Thus, the linker brings the cargo along and the result is an antibody labeled with the desired molecule. The entire antibody labeling procedure can be accomplished in under 2 hours with 30 seconds hands-on time (Fig. 2).

Fig. 2. Site-specific Antibody labeling with Photo-reactive Antibody Binding Domain Linker: The Y-shaped antibody molecule is bound to two cargo molecules with the help of the photo-reactive antibody binding domain linker and 365 nm ‘black’ light exposure. The cargo is shown as a green circle, and the linker molecule is attached. With site-specific labeling, the linker directs the cargo onto the Fc-constant region of the antibody. Up to two cargo-labels can be added with this approach shown. The chart on the right shows a highly homogeneous mixture of labeled antibodies
Another distinguishing feature of our method is its broad compatibility with a wide pH range and buffer conditions, which traditional non-specific chemical labeling methods cannot achieve. This reduces the need for optimization or purification and makes labeling “plug and play”. The result of this site-specific labeling method is a yield of homogeneous antibody conjugates that offer improved control and assay performance for a wide number of applications that utilize antibodies. This includes assays that require the immobilization of antibodies on surfaces, whereby site-specific labeling can ensure correct antibody orientation on these surfaces and dramatically improve assay sensitivity. We envision that photo-reactive antibody-binding domains will be widely useful in improving a diverse range of antibody applications.