Your cart is currently empty!
Read our feature in the Penn Bioengineering Blog here regarding the COVID-19 Grant.
NIH SBIR Phase II Grant Extension— 5-R44-EB023750-03 (PI: Yu) — 10/07/2020 – 05/30/2021
PHILADELPHIA, PA, Aug. 25, 2020 — To combat the COVID19 pandemic caused by the SARS-CoV2 virus, AlphaThera was recently awarded a SBIR Phase II Grant Extension to support its efforts in developing and commercializing COVID19 detection technology. The grant supports three major goals focused on accommodating the growing need for anti-viral antibody testing.
The need for accurate and efficient serological tests to diagnose anti-viral antibodies is crucial in establishing disease prevalence, understanding immunity, testing vaccine efficacy and monitoring disease resurgence. AlphaThera has recognized the increased demand for effective immunoassays, such as ELISA, that are needed to overcome this pandemic. Thus, AlphaThera is leveraging its expertise with conjugation technology toward achieving three goals for commercialization of COVID-19 detection products:
-
Developing a “Single-Wash” SARS-CoV2 ELISA Serology Assay enabled by AlphaThera’s proprietary photo-reactive binding domain (pAbBD) Technology
-
Developing a Highly Sensitive, no-blocking, Reusable SARS-CoV2 ELISA Serology Assay enabled by AlphaThera’s Site-Specific Immobilization Technology
-
Developing pAbBD constructs for use in “mix-and-read” Homogeneous Anti-Viral Antibody Assays
Through achieving these aforementioned goals, AlphaThera’s available products will reduce assay variability, time and costs, enabling our country to meet the increasing demand for daily testing. With partners at the Hospital of the University of Pennsylvania’s Clinical Laboratory, AlphaThera is committed to bringing its conjugation technology to the market and aiding in the fight against the pandemic.
Full Description of Project:
To combat the COVID-19 pandemic caused by the SARS-CoV2 virus, Dr. Andrew Tsourkas’s Targeted Imaging Therapeutics and Nanomedicine (Titan) Lab in Penn Bioengineering, in collaboration with the Penn-based startup, AlphaThera, was recently awarded a $667,000 SBIR Phase II Grant Extension to support its efforts in commercializing COVID-19 detection technology. The grant supports work to address the growing need for anti-viral antibody testing. Specifically, the Tsourkas Lab and AlphaThera hope to leverage their expertise with antibody conjugation technologies to reduce the steps and complexity of existing detection assays to enable greater production and higher sensitivity tests. AlphaThera was founded in 2016 by Andrew Tsourkas, PhD, Professor of Bioengineering and James Hui, MD, PhD, a graduate of the Perelman School of Medicine and Penn Bioengineering’s doctoral program.
During this pandemic it is crucial to characterize disease prevalence among populations, understand immunity, test vaccine efficacy and monitor disease resurgence. Projections have indicated that millions of daily tests will be needed to effectively control the virus spread. One important testing method is the serological assay: These tests detect the presence of SARS-CoV2 antibodies in a person’s blood produced by the body’s immune system responding to infection. Serological tests not only diagnose active infections, but also establish prior infection in an individual, which can greatly aid in forecasting disease spread and contact tracing. To perform the serological assays for antibody detection, well-established immunoassay methods are used such as ELISA.
A variety of issues have slowed the distribution of these serological assays for antibody testing. The surge in demand for testing has caused shortages in materials and reagents that are crucial for the assays. Furthermore, complexity in some of the assay formats can slow both production and affect the sensitivity of test results. Recognizing these problems, AlphaThera is leveraging its novel conjugation technology to greatly improve upon traditional assay formats.
With AlphaThera’s conjugation technology, the orientation of antibodies can be precisely controlled so that they are aligned and uniformly immobilized on assay detection plates. This is crucial as traditional serological assays often bind antibodies to plates in a non-uniform manner, which increases variability of results and reduces sensitivity. See Fig 1 below. With AlphaThera’s uniform antibody immobilization, assay specificity could increase by as much as 1000- fold for detection of a patient’s SaRS-CoV2 antibodies.
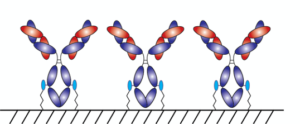
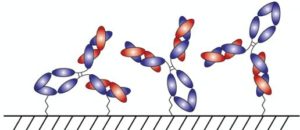
Fig 1: Uniform vs Non-Uniform Immobilized Antibodies on Surface: Left is AlphaThera improvement, showing how antibodies would be uniformly immobilized and oriented on a plate for detection. Right is how many traditional serological assays immobilize antibodies, resulting in variability of results and lower specificity