Your cart is currently empty!
There are many antibody labeling technologies available on the market that enable quick and easy conjugation to a range of labels. However, the majority of available products are not site-specific – they cannot guarantee where and how the label will be attached to the antibody.
In this blog we take a look at the impact of heterogenous labeling on antibody performance and assay results, and how these issues can be overcome with AlphaThera’s novel site-specific conjugation method.
The problems with non site-specific labeling
An antibody will typically have many lysine residues which can vary in number and location from antibody to antibody. As the majority of labeling reagents target lysine residues, this often results in the random attachment of the label at any point on the antibody and in any quantity, resulting in a heterogenous mix of antibody conjugates.
So why is heterogenous labeling a problem for researchers?
Interference with antigen binding sites leads to reduced antibody binding performance
As lysine residues can be located within the antigen binding site, using non site-specific labeling techniques can result in the label attaching within this important antigen binding region. This may interfere with the antibody binding to its target, resulting in a decrease in overall assay performance.
This can be even more of an issue when attaching large biomolecules to the antibody (e.g. enzymes), which can sterically prevent antigen binding.
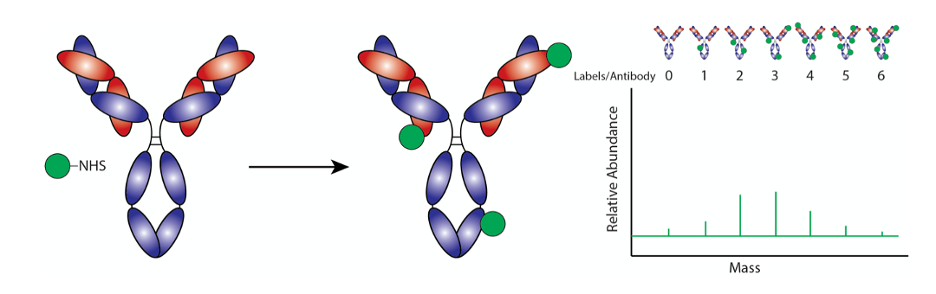
Non-uniform label distribution leads to inconsistent and unpredictable results
Heterogenous conjugates resulting from non site-specific antibody labeling make it challenging to replicate results of an assay as it is near impossible to ensure consistent and uniform distribution of labels across every antibody. This can also be an issue with assuring batch-to-batch consistency.
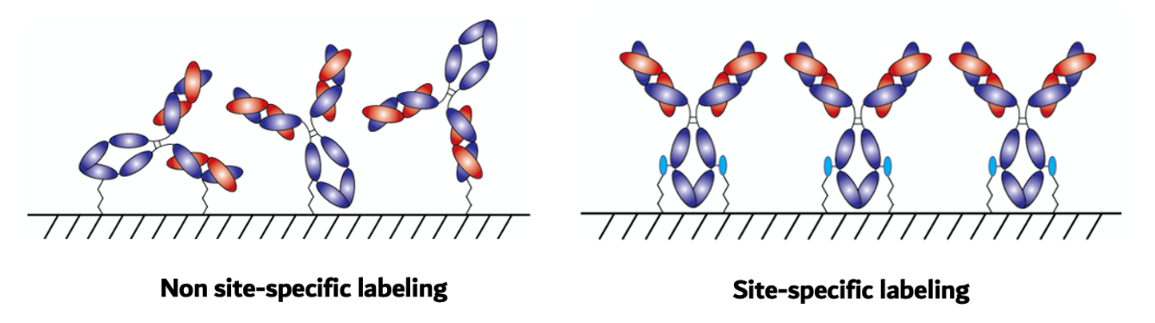
Improper orientation of immobilized antibodies
Non site-specific labeling often results in the random orientation of immobilized antibodies which can reduce the density of available antigen binding sites on the surface and in reduce the sensitivity of most immunoassays.
The need to optimize reaction conditions & time-consuming purification steps
The efficiency of non-site-specific labeling of lysines can vary dramatically from antibody to antibody. As a result, the reaction conditions may need to be optimized to achieve the desired results.
Furthermore, reagents that rely on lysine labeling typically require the removal of storage proteins and/or exchange of amine-containing buffers. This is not only time-consuming, but also can result in a significant loss of antibody.
Introducing oYo-Link® Site-Specific antibody labeling technology
Covalently label your antibody with just 30 seconds hands-on time
oYo-Link® is a revolutionary antibody labeling technology that allows quick and easy, site specific, covalent labeling of almost any “off-the-shelf” antibody with biotin, click chemistry tags (e.g. azide, DBCO), drugs, enzymes (e.g. MNase), oligonucleotides, and more.
Labeling is simple, fast, and site-specific. The entire procedure requires just two-steps and less than 30 seconds of hands-on time. The reaction is complete in just 2 hours.
How does it work?
oYo-Link® reagents consist of low molecular weight (~8 kDa), high-affinity antibody-binding domains that possess a photo-crosslinker within their Fc-binding site. Upon illumination with non-damaging Black-light, oYo-Link forms a covalent bond with the antibody. This procedure is referred to as Light-Activated Site-Specific Conjugation (LASIC). Any label that is attached to oYo-Link will be covalently attached to the desired antibody.
Advantages of site specific labeling using oYo-Link®
-
Covalent, site-specific labeling to avoid dissociation
While some other site-specific methods may claim to be rapid, these generally do not result in a covalent linkage between the label and the antibody. Therefore, the label can dissociate from the antibody during an experiment. This can result in loss of signal, increased background, or both.
-
Ensures uniform labeling of up to 2 labels per antibody on the heavy chain
Resulting in predictable and reproducible results from assay to assay.
-
Effective binding away from binding site & uniform surface immobilization
No interference with antigen binding. Ensures the proper orientation of immobilized antibodies. Proper orientation of antibodies on surfaces can result in a significant improvement in the density of available antigen binding sites on the surface and in turn improve the sensitivity of most immunoassays.
-
Antibody and buffer compatibility
The oYo-Link site-specific antibody labeling system avoids the need to optimize reaction conditions for each antibody. The same reaction conditions are used for all oYo-Link compatible antibodies and results in a predictable number of labels per antibody. A particularly notable advantage of the oYo-Link site-specific antibody labeling system is that antibody labeling can be conducted in nearly any buffer, including those containing amines (e.g. Tris) or storage proteins (see Buffer Compatibility Table).
-
Enables labeling at low Ab concentrations (as low as 50 µg/mL)
Label as little as 1 µg of antibody at a time and work with antibody concentrations as low as 50 µg/mL. Therefore, there is no need to concentrate or centrifuge your sample prior to labeling. In addition, any excess oYo-Link not used in a reaction can be stored for later use.
-
Wide range of labels available
oYo-Link enables labeling to a range of labels including drugs, biotin, click chemistry tags (e.g. azide, DBCO), enzymes (e.g. MNase), and oligonucleotides.
You can find out more about oYo-Link site specific labeling here. If you have more questions about using oYo-Link labeling technology to conjugate your antibody then please don’t hesitate to get in touch, our expert technical support team are happy to help!
Watch our video to find out more about oYo-Link antibody labeling technology.